WB单条带再论证
The short answer is potentially, but not always. A single, distinct band at the right molecular weight may represent the target antigen (protein), however, it could also be nonspecific binding to an off-target protein or both, which will ultimately produce inaccurate results (Holmseth et al., 2012; Pillai-Kastoori et al., 2020). Here is a personal experience to illustrate this point. During my postdoc training, I was working on a protein phosphatase called MAP kinase phosphatase -1 (MKP-1). At that time, there were a plethora of commercially available antibodies against MKP-1. I tested several of these commercially available MKP-1 antibodies, and most of them detected a single band at its expected molecular weight of 40 kDa. These antibodies, sold by reputable vendors, were estimated to be used in 1,000 peer-reviewed published papers. Later, our lab knocked out the Mkp1 gene (Mkp1-/-) in a mouse model and collected tissue lysates from both wild-type and Mkp1-/- mice. After running a Western blot, we found all of the commercial antibodies produced distinct bands at the predicted weight of 40kDa in both Mkp1-/- lysates as well as in control lysates. This experience taught me a hard lesson regarding antibody specificity: unless you knock out or knock down a gene, and thus its corresponding protein(s), the assumption that a single, distinct band at the right molecular weight represents your desired protein is not scientifically sound.
To further illustrate the above perspectives, I would like to use PCNA antibodies from different vendors as an example.
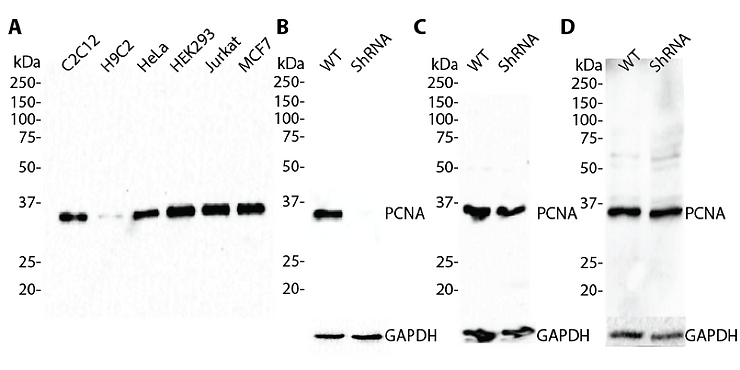
Figure 1. Western blots showing the specificity of antibodies from different vendors. (A) A total of 30 μg of whole cell lysates from different cell lines were immunoblotted using an anti-PCNA antibody (#6151) from GenuIN Biotech. (B-D) PCNA was knocked down in HeLa cells using a GenuIN Biotech customized lentiviral shRNA. Wild-type (WT) and PCNA shRNA-knockdown (shRNA) cell lysates were blotted with anti-PCNA antibodies from GenuIN Biotech (#6151) (B), vendor X (C), and vendor Y (D). GAPDH served as a loading control.
In Figure 1A, we showed the PCNA antibody from GenuIN Biotech (#6151) detected a single band at the predicted size of 36 kDa. We then designed a shRNA sequence that specifically targeted PCNA mRNA, generated shRNA lentiviral particles, and infected HeLa cells with the PCNA targeting shRNA lentiviruses. We showed that the lentiviral shRNA knockdown resulted in a loss of PCNA at the protein level, which is depicted by an absence of signal after immunoblotting (Figure 1B). These findings indicate that our PCNA antibody (#6151) bound to its intended epitope (target protein), and thus was specific. In contrast, the antibody from vendor X failed the specificity test as a result of a signal being detected despite the PCNA protein being absent in the cell lysate (Figure 1C). Additionally, we showed that the antibody from vendor Y not only detected a similar nonspecific band at the predicted size in the knockdown lysate, but also exhibited nonspecific binding with proteins of different sizes (Figure 1D). The results from vendors X and Y can easily lead scientists to assume the antibody is specific based on the single, expected band of the correct size; however, data from the knockdown lysate clearly show non-specificity.
In summary, using verification techniques such as RNA interference (knockdown; KD) (Dykxhoorn et al., 2003; Xie and Fussenegger, 2018) and CRISPR/Cas9 mediated gene targeting (knockout; KO) (Hsu et al., 2014; Shalem et al., 2014; Wang et al., 2013) to test antibody specificity has unique and apparent advantages (Pillai-Kastoori et al., 2020). It not only provides a true negative control, but also exposes antibodies that have off-target binding if a positive signal from KD/KO cell lysates is detected (Pillai-Kastoori et al., 2020). Collectively, the implementation of the KD/KO approach ensures accurate and sound data collection.
References
Dykxhoorn, D.M., Novina, C.D., and Sharp, P.A. (2003). Killing the messenger: short RNAs that silence gene expression. Nat Rev Mol Cell Biol 4, 457-467.
Holmseth, S., Zhou, Y., Follin-Arbelet, V.V., Lehre, K.P., Bergles, D.E., and Danbolt, N.C. (2012). Specificity controls for immunocytochemistry: the antigen preadsorption test can lead to inaccurate assessment of antibody specificity. J Histochem Cytochem 60, 174-187.
Hsu, P.D., Lander, E.S., and Zhang, F. (2014). Development and applications of CRISPR-Cas9 for genome engineering. Cell 157, 1262-1278.
Pillai-Kastoori, L., Heaton, S., Shiflett, S.D., Roberts, A.C., Solache, A., and Schutz-Geschwender, A.R. (2020). Antibody validation for Western blot: By the user, for the user. J Biol Chem 295, 926-939.
Shalem, O., Sanjana, N.E., Hartenian, E., Shi, X., Scott, D.A., Mikkelson, T., Heckl, D., Ebert, B.L., Root, D.E., Doench, J.G., et al. (2014). Genome-scale CRISPR-Cas9 knockout screening in human cells. Science 343, 84-87.
Wang, H., Yang, H., Shivalila, C.S., Dawlaty, M.M., Cheng, A.W., Zhang, F., and Jaenisch, R. (2013). One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell 153, 910-918.
Xie, M., and Fussenegger, M. (2018). Designing cell function: assembly of synthetic gene circuits for cell biology applications. Nat Rev Mol Cell Biol 19, 507-525.