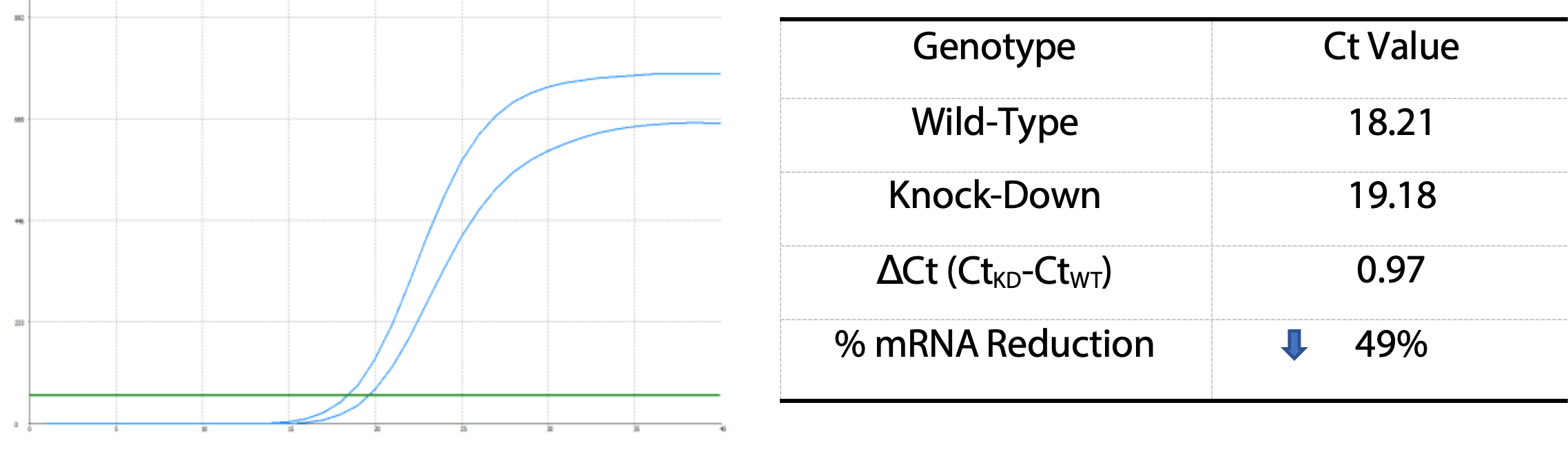
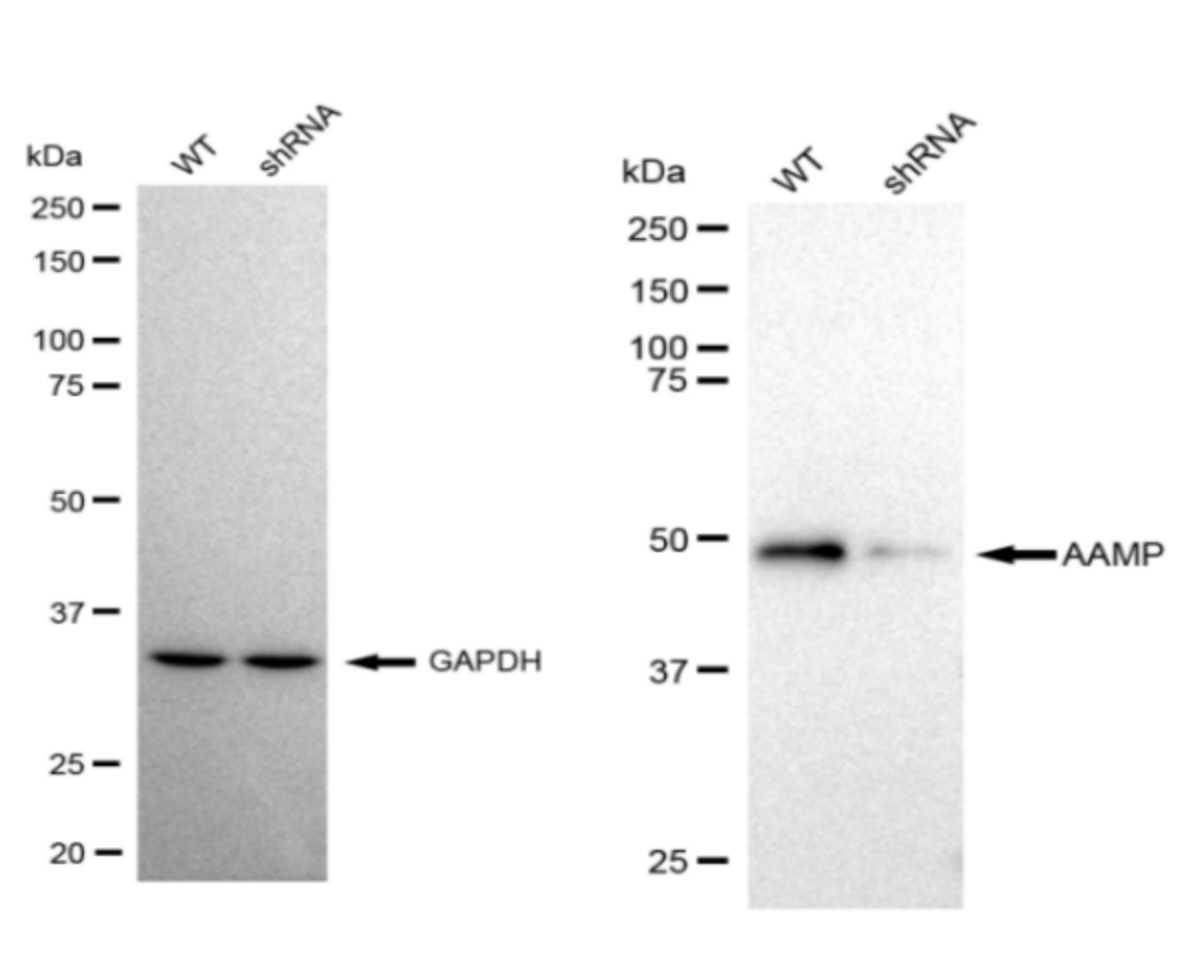
I. Identification:
Catalog Number: V61156
Product Name: WB-Validated AAMP Lentiviral shRNA Knockdown Kit #V61156
Manufacturer & Supplier: Hefei SciBen Biotech Co., Ltd
Address:Building 6, 98 Jiulong Road, Hefei, Anhui 230601, China
Tel: 540.605.9777
Fax: 540.605.9771
E-mail:support@scibenbio.com
II. Composition/Information on Ingredients:
Mixtures: No components need to be disclosed according to the applicable regulations.
III. Hazard Identification:
Classification of the substance or mixture: Not a hazardous substance or mixture.
GHS Label elements, including precautionary statements: Not a hazardous substance or mixture.
Hazards not otherwise classified (HNOC) or not covered by GHS Biohazard, Biosafety Level 2: Lentiviruses are a subset of retroviruses, able to integrate into host chromosomes, and to infect nondividing cells. More recent generation vectors have been designed to diminish possible recombination resulting in a wild type- potentially infectious virus. It is usually recommended that work with nonhuman lentiviruses that are incapable of establishing productive infections in humans be conducted at BSL-2.
IV. First Aid Measures:
Inhalation: If breathed in, move person into fresh air. If not breathing, give artificial respiration.
In case of skin contact:Wash off with soap and plenty of water.
In case of eye contact:Flush eyes with water as a precaution.
If swallowed: Never give anything by mouth to an unconscious person. Rinse mouth with water.
V. Fire Fighting Measures:
Flash Point: Data not available.
Autoignition Temperature: Data not available.
Fire Extinguishing Media:Water spray, dry chemical, foam, or carbon dioxide.
Firefighting:Wear protective clothing and self-contained breathing apparatus to prevent contact with skin and eyes.
VI. Accidental Release Measures:
Absorb liquid with an absorbent material. Transfer contaminated absorbent to a chemical waste container for disposal.
VII. Handling And Storage:
Avoid inhalation and contact with eyes and skin. Avoid prolonged or repeated exposure. Store at –20 °C in tightly closed container.
VIII. Exposure Controls/Personal Protection:
Engineering Controls:Maintain adequate ventilation, eye wash and quick-drench facilities
IX. Physical and Chemical Properties:
Physical State:liquid.
Odor:Odorless.
Boiling Point:Data not available.
Melting Point:Data not available.
Volatile Organic Compound:Data not available.
Solubility in water:Readily miscible in water.
X. Stability and Reactivity:
Reactivity: Data not available
Chemical stability:Stable under recommended storage conditions.
Possibility of hazardous reactions: Data not available.
Conditions to avoid: Data not available.
Incompatible materials:Strong oxidizing agents.
Hazardous decomposition products: Other decomposition products - Data not available
XI. Toxicological Information:
Acute toxicity: Data not available.
Inhalation: Data not available.
Dermal: Data not available.
Skin corrosion/irritation: Data not available.
Serious eye damage/eye irritation: Data not available.
Respiratory or skin sensitization: Data not available.
Reproductive toxicity: Data not available.
Aspiration hazard: Data not available.
XII. Ecological Information:
Toxicity: Data not available.
Persistence and degradability:Data not available.
Bioaccumulative potential: Data not available.
Mobility in soil: Data not available.
XIII. Disposal Considerations:
Dispose of in accordance with federal, state and local environmental regulations.
XIV. Transport Information:
D.O.T.: This substance is considered non-hazardous for transport.
IATA: This substance is considered non-hazardous for air transport.
XV. Regulatory Information:
EU Regulation/Classification/Labeling Information: Not available for this product.
Chemical Inventory Status:
SARA Listed Component: None.
TSCA Listed Component: None.
Canada (WHMIS): DSL No, NDSL No.
XVI. Other Information:
This product is sold only for research use by personnel familiar with chemicals and who are well trained in good laboratory habits, such as avoiding spills, keeping hands clean at all times and not rubbing eyes with hands while working in the laboratory.
This product is sold only in microliter quantities for use in life sciences research. No other use is intended, and any other use may involve substantive hazards.
The above information is believed to be correct but does not purport to be all inclusive and shall be used only as a guide for experienced personnel. Hefei SciBen Biotech Co., Ltd shall not be held liable for any damage resulting from the handling of or from contact with the above product. The burden of safe use of this material rests entirely with the user.
Issuing Date: 8/16/2024 Version: 1.0